Leading Minds Network: Seminar Highlights Amsterdam 2019
Leading Minds Seminars were created several years ago to facilitate discussions amongst temperature control practitioners to address common problems and to move best practices forward. We do this through highly informative expert presentations and collaborative learning that happens through the many discussions.
ELPRO and Sonoco ThermoSafe thank all the participants for attending and contributing their experiences during the seminar and User Groups.
Here we will share with you, the highlights of Leading Minds Amsterdam, November 2019, and what lessons we learned. The topics covered include:
-
Real Time Monitoring and IoT
-
Risk Management of Air Freight
-
Temperature Monitoring Software Case Study
-
Quality Control in the Last Miles
-
GDP Inspection Findings
-
Re-usable Packaging
-
Temperature Indicators
Real Time Monitoring and IoT
The Community Forum Discussion on Real Time Monitoring and IoT: Evaluating Opportunities for Realizing End to End Visibility included panellists Gino Vleugels, Senior Manager EMEA Temperature Control at Johnson & Johnson who states that in-transit visibility can be valuable to reduce the risk of high-value goods. 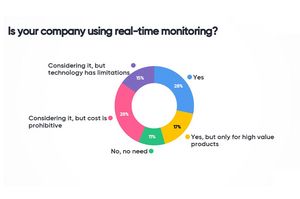
Ron Haub, Segment Director at Sonoco ThermoSafe creates the word “analysis of things”, meaning that collection of data alone is not adding value without working with it. Martin Peter, VP Strategy at ELPRO, sees the biggest opportunity in the automation of existing processes. Ruud van der Geer, Associate Director Supply Chain Management EMEA at MSD agrees to this and is convinced that IoT will help any company reduce cost.
One big challenge will be to connect the relevant data and understand it. When discussing the possibility of sharing data among the industry, the major issue is about GxP relevance of 3rd party data: sharing sensitive information may be critical for validation reason. The panelists agree that it will take additional time and experiences in order to identify the use and value of this additional visibility. It is also expected regulations will address this topic in more detail in the future.
Key Takeaways:
-
There are lots of layers of value to IoT, including reducing risk and diversion; while increasing visibility and security
-
The technology is still developing and pharma manufacturers are keen to see it advance
Risk Management of Air Freight – Mytigate Project
Professor Yvonne Ziegler, from the University of Frankfurt Applied Sciences, spoke about MYTIGATE, a project that is addressing how to reduce transportation risks by using data from across manufacturers to calculate risk in supply lanes, specifically in air freight. Working with various companies on the project team including Bayer, Boehringer Ingelheim and more – they have identified the requirements in supply chains (including legal regulations, product specifications, business regulations) and matched them with capabilities of each supply chain partner (QMS, staff training, operations, safety and security) to calculate risk. The Mytigate validated software applies risk modelling that uses a series of questions to narrow down outcomes. Professor Ziegler explained years of research has isolated temperature deviations as a main cause of risk and product loss, and how the system has the potential to integrate with liberoMANAGER, temperature management database, for further verification of lane partner.
Key Takeaway:
There is great potential for a system like Mytigate to offer detailed insight on lane assessments and risk evaluation with partners. However, there are still many requests for the system to be developed further and we look forward to those in 2020. For more information, visit www.mytigate.com.
Temperature Monitoring Software Case Study
A multi-national pharmaceutical company spoke about how they developed their risk-based approach to of the entire network, trade lanes, packaging and logistics providers. Based on this analysis, they have define their monitoring strategy including where to monitor (and not), how to monitor and how to collect the data. A key part of risk analysis strategy is whether to use "controlled equipment" (active containers, high sensitive products and always monitoring (all lanes, all shipments); or use "non-active equipment" (dry-containers, insulated containers) for more targeted, selected monitoring. Ultimately, their risk based approach results in monitoring 57% of all shipments. Benefits including costs savings on amount of data loggers and logistics services.
Part of the project success would be using the right monitoring software and hardware. After evaluating monitoring systems, the pharmaceutical company choose ELPRO’s liberoMANAGER. Key reasons include no need to install anything at origin or destination; user management allowing access for different roles; and automated notifications to manage problems with sites.
To implement a successful monitoring system, you need to be clear about the scope and the target; all stakeholders involved; a partnership with your monitoring partner providing best practice, innovation and consulting; and a strong coordination on "global level" and a strong team to manage deployment and analysis/control during operation.
Key takeaways:
Having the temperature centrally in the cloud, allowed the pharmaceutical company to analyze deviations quickly and make effective CAPAs; including:
-
Wrong packaging material applied → re-training of employees
-
Lead time was too long → improve process
-
Wrong truck used → improve process
-
Extreme weather conditions → change in equipment for this season
Quality Control in the Last Miles
This discussion brought two perspectives of the last miles. Samantha Carmichael, Lead Pharmacist Clinical Trials at NHS Glasgow receives several hundred clinical trial shipments to their hospital every month. Hok Tjiook, Pharmacist and Owner of eFarma, an online pharmacy in Holland, knows the challenges of temperature control shipments in the “very last mile” to the patient.
From the clinical perspective, Sam describes one approach with a relatively "non-sensitive" product where they have perform ed a risk analysis to conclude that monitoring was not needed. This was submitted to the authorities and was accepted. But this approach would not always be acceptable — it really depends how sensitive a product is. Also at clinical sites, we have eight different data loggers on the same fridge. Each sponsor comes with their own data logger and asks the site for proof.
ed a risk analysis to conclude that monitoring was not needed. This was submitted to the authorities and was accepted. But this approach would not always be acceptable — it really depends how sensitive a product is. Also at clinical sites, we have eight different data loggers on the same fridge. Each sponsor comes with their own data logger and asks the site for proof.
From the pharmacy perspective, cost is an issue. The patient needs to be supported, offering the ability to decide if the product is good or not. An indicator can be a good choice, but still cost is an issue. The gross margin on products needs to be considered. Similar to clinical, they also have 10 different devices monitoring refrigerators and storage facilities.
Direct to Patient (DtP) is a huge trend, and in clinical trials it’s for convenience. Patients don’t want to travel to clinical sites if they don’t have to. If trial sponsors want to increase recruiting performance, they better offer DtP.
To read more in-depth on this topic, download the whitepaper “A New Approach for Temperature Monitoring in a Changing Clinical Supply Chain Environment”.
GDP Inspection Findings
Riekert Bruinink , GMP/GDP consultant, formerly Dutch Healthcare Inspectorate, spoke about findings from 35 inspections over the past several years. The inspections were of pharmaceutical manufacturers, wholesalers, importers and secondary packaging companies. He explained that in 93% of inspections of storage monitoring facilities; monitoring equipment was installed. However, 17% were out-of-date and not suitable for use anymore; and that only 32% performed summer and winter mappings. Therefore, although monitoring systems were installed, their use and processes were not always sufficient. Mr Bruinink also spoke about working with logistics subcontractors and ensuring they are properly trained on how to handle products correctly, especially on how to handle and communicate excursions.
Key takeaways:
-
If monitoring devices alarm, ensure all personnel are training along the supply chain on how to handle alarms and protocol is in place to action them.
-
Evaluate subcontractors carefully, document your expectations, processes and their responsibilities in an SLA
-
Ensure contracts (QA/QTA) are always based on EU GDP Guidelines 2013
- Reusable Packaging

Vivian Berni, Sonoco ThermoSafe, talked about reusable shipping systems case studies and implementation models. Vivian described one of the technology changes being a move from physically handing SOP’s written on pieces of paper from one person in the chain to the next and onto today where it is all digitized. She explained how innovation is taking us forward but at the same time, technology is also driving complexity and uncertainty. Packaging as a service should help with those complexities and uncertainties.
Vivien described 4 pillars of packaging as a service being:
- Packaging and technologies
- Qualified systems
- Durable
- Ease of use - Services and Support
- Ability to outsource
- On demand flexibility - Insights o Gathering real time data
- Benefits
- Drive lower overall costs
- Impact sustainability
- Operational benefits
Key takeaways:
-
Drivers for implementing a sustainability program include reducing payload risk; reducing operational cost; and reducing environmental impact
-
Organizational challenges to adopting change can include people skills and accountability; process limitations using technology; inexistent or immature sustainability policy.
Temperature Indicators: When Do They Make Sense for Your Supply Chain
Andreas Giger, a proficient specialist with 15 years’ experience in electronic indicators, spoke about the value of using indicators versus data loggers.
In the beginning of this century, electronic indicators started to evolve. Compared to the previously common chemical indicators, electronic indicators solved a lot of issues: they have a more accurate temperature sensor, allow set multiple alarm thresholds with individual excursion allowances and can be stopped, which can be important to record a final status. Indicators have its core application in monitoring shipments to emerging markets (e.g. vaccines). The group shares additional use cases such as the monitoring of medical devices to avoid the loss of return shipments or the possibility to monitor on a box level. The latest generation of indicators helps us overcome recent challenges:
Challenge:
-
No documentation and archiving possibility of the final status
-
No information on alarm time
-
Manual, non-compliant process
-
Limited to last mile (battery life < 20 days)
-
Selective application due to size and cost
Solution:
-
Electronic read out and upload possibility to a data repository
-
Additional statistics such as alarm time
-
Automated, compliant process
-
end-to-end visibility over the complete lifetime of a product (several years)
-
cost-efficient application on a box level
Andreas Giger summarizes, that this allows many new application areas, e.g. the monitoring on a box level over a whole product life. Important is to mention, that an indicator does not replace a datalogger functionality as it does not provide a temperature curve.
In closing:
Overall, the seminar was very active and engaging. The audience live poll during the morning icebreaker session indicated a number of interests by the audience, as show below in the word cloud.

Impressions of LMS 2019 in Amsterdam







Leave a Comment